Our World Class Services
The biggest challenge posed to initiate the trail quicker, constitutes of the monitoring processes that are used before the clinical study. Pharma Research Therapeutics is capable of providing regulatory services that assist with all stages of development of drug including the Phases II-IV, BE and device studies. The experience and understanding that Pharma Research Therapeutics has in regards to Indian drug control regulations and dealing with them, helps us in assisting the sponsors with procurement of regulatory approvals, better. We provide services that range from customized services to a packaged study management.
Our services include, but are not limited to:
- Registration of products for marketing approval
- Import license of drugs
- Regulatory database compilation
- Safety reports
- Regulatory approvals for different stages of drug deve

Pharma Research, provides exceedingly reliable inputs for the intend of safety management plans that are complete study specific, with the help of a highly committed and experienced group of Safety Physicians, thereby ensuring there is a regular and accurate reporting and processing of AE’s & SAEs, with compliance to the regulatory guidelines.
The safety management services of Pharma Research include:

There are many ways to create first-rate data with an aim to support the safety and effectiveness claims of a product. The resources are required at potentially huge number of sites. The medical monitoring team at Pharma Research takes on these responsibilities, thereby helping you in finding investigator site support and monitoring that is faster and more efficient.
Every clinical trial depends highly on Monitors and the monitors at Pharma Research
- Ensure compliance of protocols
- Ensure precision and dependability of clinical data
- Apply applicable regulatory requirements, GCP and SOPs
- Protect the rights, security and health of your clinical trial subjects
- Support fast recruitment
- Oversee the advancement of your clinical trial
- Help to increase approval of clinical data by the regulatory authorities

Reproductive Endocrinology
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been the industry’s standard dummy text
ever since the 1500s, when an unknown printer took a galley of type and scrambled it to make a type specimen book.
Neurology
standard dummy text ever since the 1500s, when an unknown printer took a galley of a type specimen book. type and
scrambled it to make.
For disabled
It is a long established fact that a reader will be distracted by the readable content of a page when looking at its
layout. The point of using Lorem Ipsum.
Any successful clinical trial depends highly on amethodical feasibility and site selection. At Pharma Research, the center of our proposal activity and project planning is the feasibility process. Almost every area of the medical sector has been worked on by the feasibility group at Pharma Research. We also are in direct contact with the trail sites and important investigators.
The feasibility assessment process that we follow at Pharma Research ensures, proficient identification of finest investigative sites that are worth conductingclinical trial, and thus provide highly effective results.
Our dedicated team has vast experience in coordinating phase II to IV trials on both national and international scales. We can perform all stages of clinical trials – from feasibility to final study report – to the highest quality standards.
We have wide therapeutic experience, excellent cooperation with site staff, and in-depth knowledge of local requirements. Our core expertise and focus is in the South and South East Asia, however we are able to cover the whole of Asia and many other countries through our partner CROs. Our resourcing model provides both the flexibility and local knowledge of each country that the study requires.
Monitor plays a significant role to ensure the project success. As a company commitment to Clinical Research Excellence, we designed the role of our monitors to go beyond, than simply meeting the technical accuracy demanded by the research.
Our CRAs are trained to predict site management issues and proactively develop and implement solutions. CRAs undergo a thorough instructional regimen and are trained. This approach is employed from the earliest stages and throughout the conduct of the clinical study, to ensure the proper flow and responsiveness of the process.
All our monitors are full-time bilingual employees, equipped with laptops and are always available through mobile phones and internet when they are off site.
Whether helping your organization handle an overload of work on a single project or designing a full-service clinical program for your product, Croissance clinical trials management team has experience and knowledgeable staff members ready to assist you.
- Clinical services custom-tailored to fit your needs.
- Study document development.
- CRA management and quality control.
- Clinical monitoring and site management.
- Clinical Trial Management System.
- Investigator meeting planning.
- Third party vendor management.
- Audits and preparation for audits.
- Training.
- Patient recruitment and retention strategies.
- Feasibility studies.
- Quality training of clinical associates.
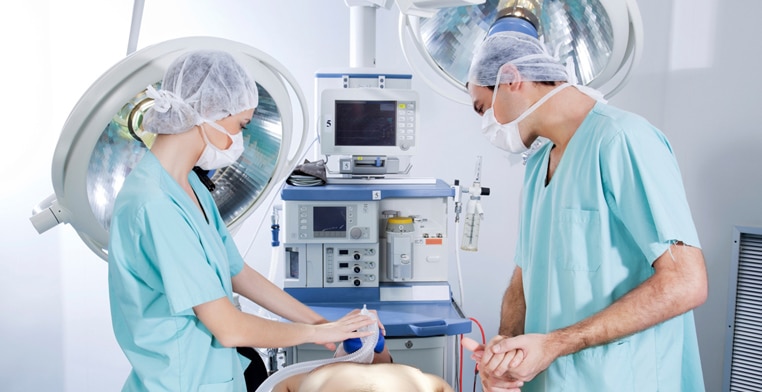
At Pharma Research, we hold the faith that efficient leaders holding the wheels in the front seat, is highly crucial for the success of any project. And so, we make sure a group of highly experienced, skilled and trained personnel complete the project management team at Pharma Research.
The basic anxiety of the customers in relation to medical trials within a specified timeline and budget is one of the top priorities for the project managers at Pharma Research Life Sciences, who make sure they have a detailed mitigation plan and risk management.
The basic functions include:
Site Monitoring
Study Feasibility
Clinical Trial Supply Management
Site and Investigator Selection
